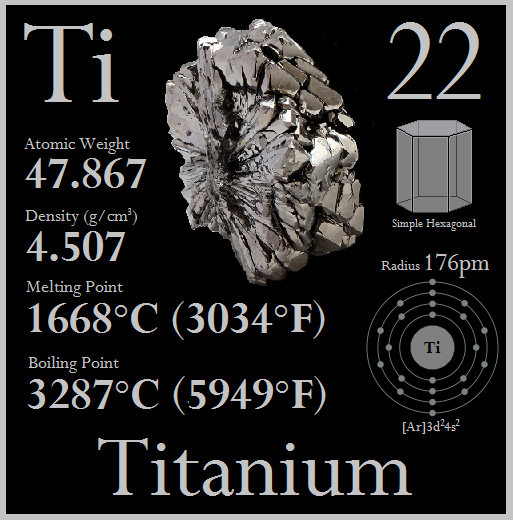
Titanium is a chemical element with the symbol Ti and atomic number 22. It has the highest strength-to-density ratio of any metallic element and is highly resistant to corrosion even when subjected to things like sea water, dilute acids, and chlorine.
Titanium oxide was discovered in 1791 by William Gregor of Cornwall and reported in the Royal Geological Society of Cornwall. It was independently rediscovered in 1795 by Prussian chemist Martin Heinrich who named it for the Titans of Greek mythology.
The processes required to extract titanium from its various ores are laborious and costly; it is not possible to reduce the ore in the normal manner, by heating in the presence of carbon, as that produces titanium carbide. Pure metallic titanium (99.9%) was first prepared in 1910 by Matthew A. Hunter by using the volatile method of heating titanium tetrachloride with sodium at 700–800°C in the Hunter process. Titanium metal was not used outside the laboratory until 1932 when William Justin Kroll proved that it could be produced by reduction with calcium. He later refined the process by using magnesium or sodium in what became known as the Kroll process. The Kroll process is still used today, though cheaper processes are still being researched.
In the 1950s and 1960s the Soviet Union pioneered the use of titanium in military and submarine applications as part of programs related to the Cold War. Starting in the early 1950s, titanium began to be used extensively for military aviation purposes, particularly in high-performance jets.
Titanium is a strong metal with low density that is quite ductile (especially in an oxygen-free environment), lustrous, and metallic-white in color. The relatively high melting point (over 1,650°C) makes it useful as a refractory metal. It is paramagnetic and has fairly low electrical and thermal conductivity.
Commercially pure grades of titanium have ultimate tensile strength of about 434 MPa (63,000 psi), equal to that of common, low-grade steel alloys, but are less dense. Titanium is 60% more dense than aluminium, but more than twice as strong as the most commonly used aluminium alloy. Certain titanium alloys achieve tensile strengths of over 1400 MPa (200,000 psi). However, titanium loses strength when heated above 430°C.
Titanium metal and its alloys oxidize immediately upon exposure to air, but it forms a passive oxide coating that protects the bulk metal from further oxidation. Related to its tendency to form a passivating layer, titanium exhibits excellent resistance to corrosion. It is almost as resistant as platinum, capable of withstanding attack by dilute sulfuric and hydrochloric acids as well as chloride solutions, and most organic acids.
Titanium is thermodynamically a very reactive metal. It burns before its melting point is reached under normal condition, making objects of titanium expensive because it cannot be cast in normal conditions; melting is only possible in an inert atmosphere or in a vacuum.
Titanium alloys have very high tensile strength and toughness (even at extreme temperatures). They are light in weight, have extraordinary corrosion resistance and the ability to withstand extreme temperatures. However, the high cost of both raw materials and processing limit their use. There are 38+ grades of titanium alloy.
Although "commercially pure" (CP) titanium has acceptable mechanical properties and has been used for orthopedic and dental implants, for most applications titanium is alloyed with small amounts of aluminium and vanadium, typically 6% and 4% respectively, by weight. This mixture has a solid solubility which varies dramatically with temperature, allowing it to undergo precipitation strengthening. This heat treatment process is carried out after the alloy has been worked into its final shape but before it is put to use, allowing much easier fabrication of a high-strength product.
Grade 5, also known as Ti6Al4V, Ti-6Al-4V or Ti 6-4, is the most commonly used alloy. It has a chemical composition of titanium, 6% aluminium, 4% vanadium, 0.25% (maximum) iron, and 0.2% (maximum) oxygen. It is significantly stronger than commercially pure titanium while having the same stiffness and thermal properties (excluding thermal conductivity, which is about 60% lower in Grade 5 Ti than in CP Ti). Among its many advantages, it is heat treatable. This grade is an excellent combination of strength, corrosion resistance, weld and fabricability.
Generally, Ti-6Al-4V is used in applications up to 400°C. It has a density of roughly 4420 kg/m3 and tensile strength of 1000 MPa (145,000 psi). By comparison, annealed type 316 stainless steel has a density of 8000 kg/m3 and tensile strength of only 570 MPa. Tempered 6061 aluminium alloy has a density of 2700 kg/m3 and tensile strength of 310 MPa.

Titanium may be anodized to produce various colors, which varies the thickness of the surface oxide layer. The colors are beautiful and show a subtle reflective nature that is specific to titanium, though they are less brilliant than niobium or aluminum. Exact colors and shades are difficult to reproduce and will vary when anodized at different times. Available colors include bronze, light and dark blue, gold, green, purple, rose, teal, and violet.
Information was collected and revised from Wikipedia and TheRingLord. Source details found at their respective sites.